- BIOGEOCHEMICAL CYCLES
- WATER CYCLE
- CARBON CYCLE
- NITROGEN CYCLE
- SULPHUR CYCLE
- PHOSPHOROUS CYCLE
UNIT 1 – ECOLOGY & ECOSYSTEM – PART 5
BIOGEOCHEMICAL CYCLES
Every life form needs to actively uptake some elements called nutrients to maintain their biological activity. These nutrients provide the structural framework of organism and energy through biochemical energy process. Few of them are essential nutrients and without their supply growth cannot take place in an organism. When these nutrients are part of organism or environment, they are called as in their biotic phase or abiotic phase respectively.
Certainly, by passing through different phases these nutrients complete closed circuit cyclic movement as Earth is a closed system. This closed-circuit movement/transformation of nutrients from geochemical to biological and to geochemical is called as biogeochemical cycle of nutrients. The continuation of these cycles is dependent on a sustained energy supply into the system. Most of them is coming from sun and remaining from earth’s internal energy.
The Earth has a finite quantity of chemical elements from its formation. Because the chemicals on Earth function in a closed system, neither significantly increasing nor decreasing in quantity, they are recycled throughout the Earth’s biological and geological cycles.
These cycles include both the living biosphere, and the nonliving lithosphere, atmosphere, and hydrosphere.
Plants absorb nutrients from soil and water, and in some cases, from the atmosphere. Nutrient cycle involves the movement and exchange of organic and inorganic matter back into the production of living matter.
The process is regulated by food web pathways that decompose matter into mineral nutrients. Nutrient cycles occur within ecosystems.
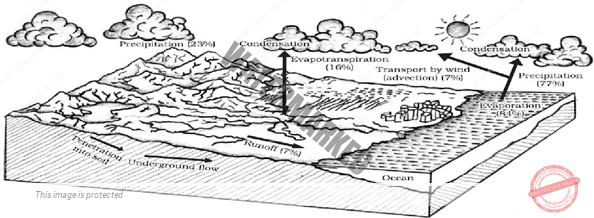
- WATER CYCLE
Precipitation is a vital component of how water moves through Earth’s Water Cycle, Connecting the Ocean, Land, and Atmosphere.
Knowing where it rains, how much it rains and the character of the falling rain, snow or hail allows scientists to better understand precipitation’s impact on Streams, Rivers, Surface Runoff and Groundwater.
Frequent and detailed measurements help scientists make models of and determine changes in Earth’s water cycle.
The water cycle describes how water evaporates from the surface of the earth, rises into the atmosphere, cools and condenses into rain or snow in clouds, and falls again to the surface as precipitation.
The water falling on land collects in rivers and lakes, soil, and porous layers of rock, and much of it flows back into the oceans, where it will once more evaporate. The cycling of water in and out of the atmosphere is a significant aspect of the weather patterns on Earth.
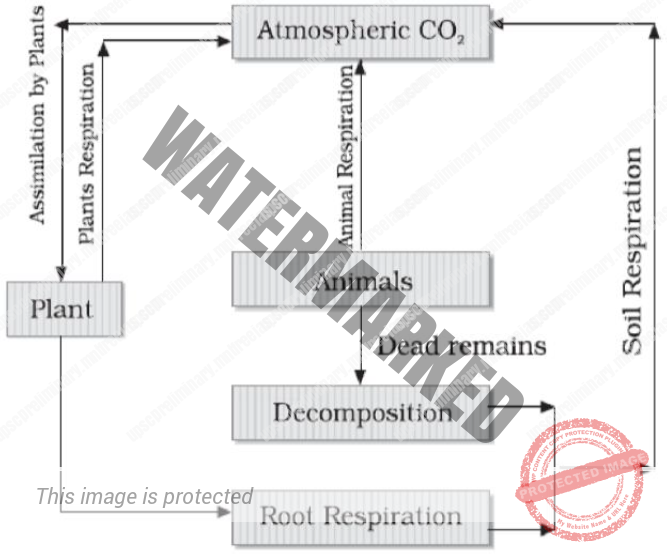
CARBON CYCLE
Carbon cycle is mainly the conversion of carbon dioxide. This conversion is initiated by the fixation of carbon dioxide from the atmosphere through photosynthesis. Such conversion results in the production of carbohydrate, glucose that may be converted to other organic compounds. Some of the carbohydrates are utilized directly by the plants itself. During the process, more Carbon dioxide is generated and is released through its leaves or roots during the day.
The remaining carbohydrates not being utilized by the plant become part of the plant tissue. Plant tissues are either being eaten by the herbivorous animals or get decomposed by the microorganisms.
The herbivores convert some of the consumed carbohydrates into carbon dioxide for release into the air through respiration. The micro-organisms decompose the remaining carbohydrates after the animal dies. The carbohydrates that are decomposed by the micro-organisms then get oxidized into carbon dioxide and are returned to the atmosphere.
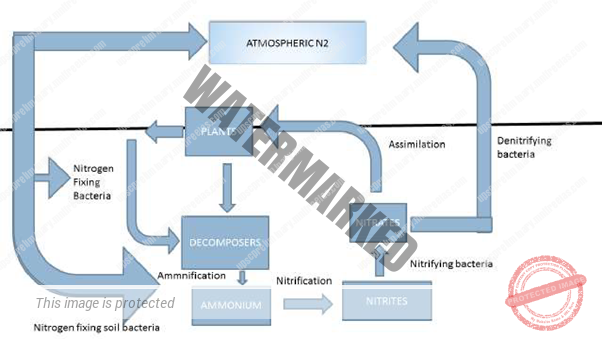
NITROGEN CYCLE
Nitrogen is one of the primary nutrients essential for the life or survival of all living organisms.
It is an essential component of many biomolecules, including fundamental amino acids, DNA, RNA, protein, base pair in nucleic acids, and chlorophyll. Although nitrogen is very abundant in the atmosphere as Dinitrogen Gas (N2), it is largely inaccessible in this form to most organisms, making nitrogen a scarce resource and often limiting primary productivity in many ecosystems. Their accessible or absorbable form is Ammonia (NH3) Or Nitrate.
The major transformations of nitrogen are Nitrogen Fixation, Nitrification, Denitrification, And Ammonification. T
he transformation of nitrogen into its many oxidation states is key to productivity in the biosphere and is highly dependent on the activities of a diverse assemblage of microorganisms, such As Bacteria, Archaea, And Fungi.
Steps involved in Nitrogen Cycle:
To convert the atmospheric nitrogen into usable nitrogen, five steps are involved. These are Nitrogen Fixation, Nitrification, Denitrification, And Ammonification.
- NITROGEN FIXATION:
Nitrogen fixation is the process by which gaseous nitrogen (N2) is converted to ammonia (NH3 or NH4 +) via biological fixation or nitrate (NO3-) through high-energy physical processes.
N2 is extremely stable, and a great deal of energy is required to break the bonds that join the two N atoms. N2can be converted directly into NO3 – through processes that exert a tremendous amount of heat, pressure, and energy.
Such processes include combustion, volcanic action, lightning discharges, and industrial means. However, a greater amount of biologically available nitrogen is naturally generated via the biological conversion of N2 to NH3/ NH4+.
A small group of bacteria and cyanobacteria are capable using the enzyme nitrogenase to break the bonds among the molecular nitrogen and combine it with hydrogen. Nitrogenase only functions in the absence of oxygen. The exclusion of oxygen is accomplished by many means. Some bacteria live beneath layers of oxygen-excluding slime on the roots of certain plants.
The most important soil dwelling bacteria, Rhizobium, live in oxygen-free zones in nodules on the roots of legumes and some other woody plants.
- NITRIFICATION:
Nitrification is a two-step process in which NH3/ NH4+ is converted to NO3–. Firstly, the soil bacteria, Nitrosomonas and Nitrococcus convert NH3 to NO2–, and then another soil bacterium, Nitrobacter, oxidizes NO2 – to NO3–. These bacteria gain energy through these conversions, both of which require oxygen to occur.
2NH4+ + 3O2 → 2NO2– + 2H2O + 4H+(Nitrosomonas)
2NO2– + O2 → 2NO3– (Nitrobacter)
Plants and animals incorporate the NO3- and ammonia formed through nitrogen fixation and nitrification. Plants take up these forms of nitrogen through their roots, and incorporate them into plant proteins and nucleic acids
DENITRIFICATION:
Denitrification is the reduction of NO3– to gaseous N2 by anaerobic bacteria. This process only occurs where there is little to no oxygen, such as deep in the soil near the water table.
Hence, areas such as wetlands provide a valuable place for reducing excess nitrogen levels via denitrification processes.
Denitrification is the only nitrogen transformation that removes nitrogen from ecosystem (essentially irreversibly) and roughly balances the amount of nitrogen fixed by the nitrogen fixers.
NO3– →N2 + N20
NO3– →NO2-→ NO →N20 →N2
- AMMONIFICATION:
Assimilation produces large quantities of organic nitrogen, including proteins, amino acids, and nucleic acids.
Ammonification is the conversion of organic nitrogen into ammonia.
The ammonia produced by this process is excreted into the environment and is then available for either nitrification or assimilation
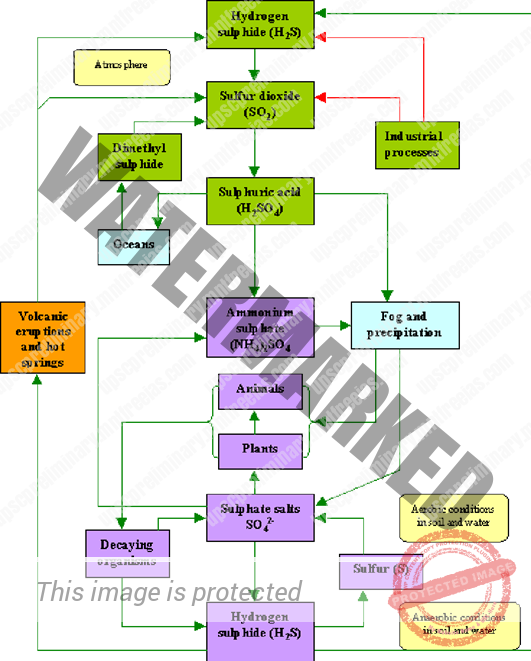
SULPHUR CYCLE
Sulphur is one of the components that make up proteins and vitamins. Proteins consist of amino acids that contain Sulphur atoms. Sulphur is important for the functioning of proteins and enzymes in plants, and animals. Plants absorb Sulphur when it is dissolved in water. Animals consume these plants; so that they take up enough Sulphur to maintain their health.
Most of the earth’s Sulphur is tied up in rocks and salts or buried deep in the ocean in oceanic sediments. Sulphur can also be found in the atmosphere.
It enters the atmosphere through both natural and human sources. Natural recourses can be for instance volcanic eruptions, evaporation from water, or decaying organisms. Sulphur enters the atmosphere through anthropogenic activity which is mainly a consequence of industrial processes where Sulphur dioxide (SO2) and hydrogen Sulphide (H2S) gases are emitted on a wide scale.
When Sulphur dioxide enters the atmosphere, it reacts with oxygen to produce Sulphur trioxide gas (SO3), or with other chemicals in the atmosphere, to produce Sulphur salts. Sulphur dioxide may also react with water to produce Sulphuric acid (H2SO4). Sulphuric acid may also be produced from dimethylsulphide, which is emitted to the atmosphere by plankton species.
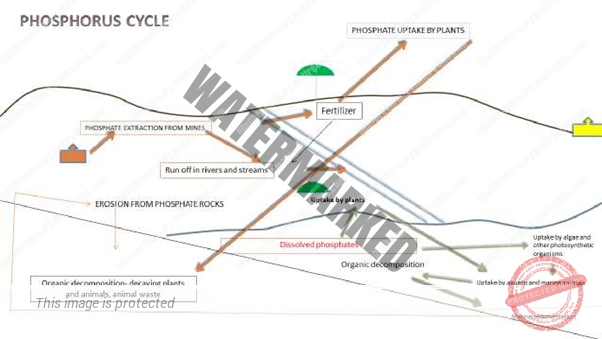
PHOSPHOROUS CYCLE
Phosphorus is an essential nutrient for plants and animals in the form of ions PO43- and HPO42-. It makes up an important part of DNA, RNA molecules that store energy (ATP and ADP) and of fats of cell membranes. Phosphorus is also a building block of certain parts of the human and animal body, such as bones and teeth.
Phosphorus can be found on earth in water, soil and sediments. Unlike the compounds of other matter cycles phosphorus cannot be found in air in the gaseous state. This is because phosphorus is usually liquid at normal temperature and pressure. It is mainly cycling through water, soil and sediments. In the atmosphere phosphorus can mainly be found as very small dust particles.
Phosphorus moves slowly from deposits on land and in sediments, to living organisms, and then much more slowly back into the soil and water sediment. Phosphorus is most commonly found in rock formations and ocean sediments as phosphate salts.
That is why humans often apply phosphate fertilizers on farmland. Phosphates are also limiting factors for plant growth in marine ecosystem because they are not very water-soluble. When animals and plants die, phosphate will return to the soil or oceans again during their decay. After that, phosphorus will end up in sediments or rock formation again and remain there for millions of years. Eventually, phosphorus is released again through weathering and the cycle starts over.