- ACID RAIN
- FORMS OF ACID DEPOSITION
- AEROSOL
- IMPACT AND HAZARD OF AIR POLLUTION
UNIT 4 – ENVIRONMENTAL DEGRADATION – PART 4
2. ACID RAIN
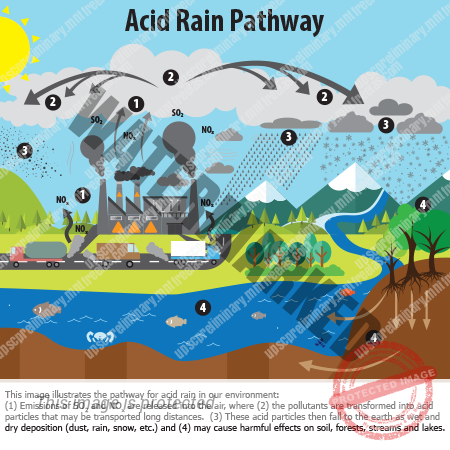
Acid Rain, Or Acid Deposition, is a broad term that includes any form of precipitation with acidic components, such as sulfuric or nitric acid that fall to the ground from the atmosphere in wet or dry forms. This can include rain, snow, fog, hail or even dust that is acidic. Normal rain has a pH of about 5.6; it is slightly acidic because carbon dioxide (CO2) dissolves into it forming weak carbonic acid. Acid rain usually has a pH between 4.2 and 4.4.
Acid rain results when Sulphur dioxide (SO2) and nitrogen oxides (NOX) are emitted into the atmosphere and transported by wind and air currents. The SO2 and NOX react with water, oxygen and other chemicals to form sulfuric and nitric acids. These then mix with water and other materials before falling to the ground.
While a small portion of the SO2 and NOX that cause acid rain is from natural sources such as volcanoes, most of it comes from the burning of fossil fuels. The major sources of SO2 and NOX in the atmosphere are:
- Burning of fossil fuels to generate electricity. Two thirds of SO2and one fourth of NOX in the atmosphere come from electric power generators.
- Vehicles and heavy equipment.
- Manufacturing, oil refineries and other industries.
Winds can blow SO2 and NOX over long distances and across borders making acid rain a problem for everyone and not just those who live close to these sources.
FORMS OF ACID DEPOSITION
WET DEPOSITION – Wet deposition is what we most commonly think of as acid rain. The sulfuric and nitric acids formed in the atmosphere fall to the ground mixed with Rain, Snow, Fog, Or Hail.
DRY DEPOSITION – Acidic particles and gases can also deposit from the atmosphere in the absence of moisture as dry deposition. The acidic particles and gases may deposit to surfaces (water bodies, vegetation, buildings) quickly or may react during atmospheric transport to form larger particles that can be harmful to human health. When the accumulated acids are washed off a surface by the next rain, this acidic water flows over and through the ground, and can harm plants and wildlife, such as insects and fish.
The amount of acidity in the atmosphere that deposits to earth through dry deposition depends on the amount of rainfall an area receives. For example, in desert areas the ratio of dry to wet deposition is higher than an area that receives several inches of rain each year.
Acid rains are increasingly becoming a life –threatening phenomena frequently occurring all over the world especially in the industrial and developing countries like India. It poses major threat to the plant and human life and effect the balance and equilibrium of the ecosystem. It has to be controlled by controlling the sources like cutting down the vehicular pollution and following industrial emissions standards.
3. AEROSOL
Aerosol is defined as a suspension of solid or liquid particles in a gas or air. They include a wide range of phenomena such as DUST, FUME, SMOKE, MIST, FOG, HAZE, CLOUDS, AND SMOG. Like the smoke from cigarettes, fumes from chimneys, dust raised by the wind and so on, are all aerosols.
Aerosols usually remain suspended in air for at least a few seconds and in some cases a year or more. Particle size of aerosol ranges from about 0.002 µm to more than 100 µm.
Atmospheric aerosol particles originate from a wide variety of natural and anthropogenic sources. Aerosols are divided into two classes, namely primary aerosols and secondary aerosols, according to the mechanisms of their formation.
- Primary aerosol: These particles are emitted directly into the atmosphere as liquid or solid form and come from combustion or fragmentation processes, for example- sea-salt, mineral aerosols (or dust), volcanic dust, smoke and soot, some organics, biological materials (plant fragments, microorganisms, pollen, etc.).
- Secondary aerosol: The particles that are formed in the atmosphere by gas-to-particles conversion processes are secondary aerosols. The new particles are formed by nucleation and condensation of gaseous precursors for example- sulphates, nitrates, and some organics.
The aerosols are regularly form and introduced in the earth’s atmosphere, undergo various physical and chemical interactions and transformations (such as coagulation, restructuring, gas uptake and chemical reaction etc.), and play a key role in a number of global processes such as:
- They serve as condensation nuclei and help in formation of clouds
- They affect the abundance and distribution of atmospheric trace gases by heterogeneous chemical reactions and other multiphase processes.
- They can scatter, absorb and emit electromagnetic radiation.
- They serve as media upon which chemical reactions can occur. 5. Airborne particles play an important role in the spreading of biological organisms, reproductive materials, and pathogens (pollen, bacteria, spores, viruses, etc.), and they can cause or enhance respiratory, cardiovascular, infectious, and allergic diseases
A significant fraction of the atmospheric aerosols is anthropogenic in origin. Aerosols are responsible for climate change, visibility reduction, and acid deposition and affect air quality and human health.
IMPACT AND HAZARD OF AIR POLLUTION
Air pollution kills an estimated seven million people worldwide every year. WHO data shows that 9 out of 10 people breathe air containing high levels of pollutants. From smog hanging over cities to smoke inside the home, air pollution poses a major threat to health and climate. The combined effects of ambient (outdoor) and household air pollution cause about seven million premature deaths every year, largely as a result of increased mortality from Stroke, Heart Disease, Chronic Obstructive Pulmonary Disease, Lung Cancer And Acute Respiratory Infections.
More than 80% of people living in urban areas that monitor air pollution are exposed to air quality levels that exceed WHO guideline limits, with low- and middle-income countries suffering from the highest exposures, both indoors and outdoors.
- AMBIENT AIR POLLUTION (OUTDOOR)
Ambient air pollution accounts for an estimated 4.2 million deaths per year due to stroke, heart disease, lung cancer and chronic respiratory diseases.
Around 91% of the world’s population live in places where air quality levels exceed WHO limits. While ambient air pollution affects developed and developing countries alike, low- and middle-income countries experience the highest burden, with the greatest toll in the WHO Western Pacific and South-East Asia regions.
The major outdoor pollution sources include vehicles, power generation, building heating systems, agriculture/waste incineration and industry. Policies and investments supporting cleaner transport, energy-efficient housing, power generation, industry and better municipal waste management can effectively reduce key sources of ambient air pollution.
Air quality is closely linked to earth’s climate and ecosystems globally. Many of the drivers of air pollution (i.e. combustion of fossil fuels) are also sources of high CO2 emissions. Policies to reduce air pollution, therefore, offer a “win–win” strategy for both climate and health, lowering the burden of disease attributable to air pollution, as well as contributing to the near- and long-term mitigation of climate change.
- INDOOR AIR POLLUTION
Household air pollution is one of the leading causes of disease and premature death in the developing world. Exposure to smoke from cooking fires causes 3.8 million premature deaths each year, mostly in low- and middle-income countries. Burning fuels such as dung, wood and coal in inefficient stoves or open hearths produces a variety of health-damaging pollutants, including Particulate Matter (PM), Methane, Carbon Monoxide, Polyaromatic Hydrocarbons (PAH) And Volatile Organic Compounds (VOC).
Burning kerosene in simple wick lamps also produces significant emissions of fine particles and other pollutants. Particulate matter is a pollutant of special concern. Many studies have demonstrated a direct relationship between exposure to PM and negative health impacts. Smaller-diameter particles (PM2.5 or smaller) are generally more dangerous and ultrafine particles (one micron in diameter or less) can penetrate tissues and organs, posing an even greater risk of systemic health impacts.
Exposure to indoor air pollutants can lead to a wide range of adverse health outcomes in both children and adults, from respiratory illnesses to cancer to eye problems. Members of households that rely on polluting fuels and devices also suffer a higher risk of burns, poisonings, musculoskeletal injuries, and accidents.
EFFECTS OF AIR POLLUTION
On human health – Short-term effects, which are temporary, include illnesses such as pneumonia or bronchitis. They also include discomfort such as irritation to the nose, throat, eyes, or skin. Air pollution can also cause Headaches, Dizziness, And Nausea. Bad smells made by factories, garbage, or sewer systems are considered air pollution, too. These odors are less serious but still unpleasant.
Long-term effects of air pollution can last for years or for an entire lifetime. They can even lead to a person’s death. Long-term health effects from air pollution include heart disease, lung cancer, and respiratory diseases such as emphysema. Air pollution can also cause long-term damage to people’s nerves, brain, kidneys, liver, and other organs.
Some scientists suspect air pollutants cause birth defects. People react differently to different types of air pollution. Young children and older adults, whose immune systems tend to be weaker, are often more sensitive to pollution. Conditions such as asthma, heart disease, and lung disease can be made worse by exposure to air pollution. The length of exposure and amount and type of pollutants are also factors.
On environment – Air pollution particles eventually fall back to Earth. Air pollution can directly contaminate the surface of bodies of water and soil. This can kill crops or reduce their yield. It can kill young trees and other plants. Sulphur dioxide and nitrogen oxide particles in the air, can create acid rain when they mix with water and oxygen in the atmosphere. These air pollutants come mostly from coal-fired power plants and motor vehicles.
When acid rain falls to Earth, it damages plants by changing soil composition; degrades water quality in rivers, lakes and streams; damages crops; and can cause buildings and monuments to decay.