- COMPOSITION OF THE ATMOSPHERE
- COMPOSITION OF AIR
UNIT 4 – CLIMATOLOGY – PART 1
COMPOSITION OF THE ATMOSPHERE
Introduction:
Earth is the only planet in the solar system with an atmosphere that can sustain life. The blanket of gases not only contains the air that we breathe but also protects us from the blasts of heat and radiation emanating from the sun. It warms the planet by day and cools it at night.
Earth’s atmosphere is about 300 miles (480 kilometers) thick, but most of it is within 10 miles (16 km) the surface. Air pressure decreases with altitude. At sea level, air pressure is about 14.7 pounds per square inch (1 kilogram per square centimeter). At 10,000 feet (3 km), the air pressure is 10 pounds per square inch (0.7 kg per square cm). There is also less oxygen to breathe.
COMPOSITION OF AIR
The gases in Earth’s atmosphere include:
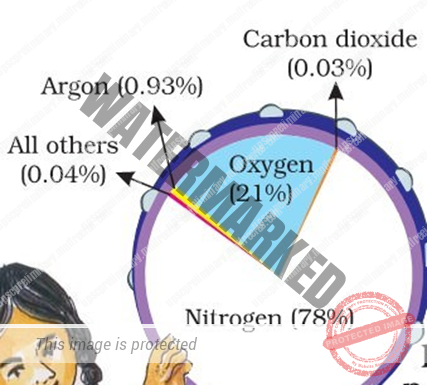
- Nitrogen — 78 percent
- Oxygen — 21 percent
- Argon — 0.93 percent
- Carbon dioxide — 0.04 percent
- Trace amounts of neon, helium, methane, krypton and hydrogen, as well as water vapor
The atmosphere is composed of gases, water vapour and dust particles. The proportion of gases changes in the higher layers of the atmosphere in such a way that oxygen will be almost in negligible quantity at the height of 120 km. Similarly, carbon dioxide and water vapour are found only up to 90 km from the surface of the earth.
Nitrogen and oxygen are two gases which make up the bulk of the atmosphere. Carbon dioxide, helium, ozone, argon and hydrogen are found in lesser quantities.
Nitrogen is the most plentiful gas in the air. When we inhale, we take some amount of nitrogen into our lungs and exhale it. But plants need nitrogen for their survival.
They cannot take nitrogen directly from the air. Bacteria, that live in the soil and roots of some plants, take nitrogen from the air, and change its form so that plants can use it
CARBON DIOXIDE is another important gas. Green plants use carbon dioxide to make their food and release oxygen. Humans or animals release carbon dioxide.
CARBON DIOXIDE is meteorologically a very important gas as it is transparent to the incoming solar radiation but opaque to the outgoing terrestrial radiation. It absorbs a part of terrestrial radiation and reflects back some part of it towards the earth’s surface. It is largely responsible for the greenhouse effect.
OZONE is another important component of the atmosphere found between 10 and 50 km above the earth’s surface and acts as a filter and absorbs the ultra-violet rays radiating from the sun and prevents them from reaching the surface of the earth.
OXYGEN is the second most plentiful gas in the air. Humans and animals take oxygen from the air as they breathe. Green plants produce oxygen during photosynthesis. In this way oxygen content in the air remains constant.
WATER VAPOUR
Water vapour is also a variable gas in the atmosphere, which decreases with altitude. In the warm and wet tropics, it may account for four per cent of the air by volume. while in the dry and cold areas of desert and polar regions, it may be less than one per cent of the air.
Water vapour also decreases from the equator towards the poles. It also absorbs parts of the insolation from the sun and preserves the earth’s radiated heat. It thus, acts like a blanket allowing the earth neither to become too cold nor too hot.
Water vapour also contributes to the stability and instability in the air.
DUST PARTICLES
which may originate from different sources and include sea salts, fine soil, smoke-soot, ash, pollen, dust and disintegrated particles of meteors.
Dust particles are generally concentrated in the lower layers of the atmosphere; The higher concentration of dust particles is found in subtropical and temperate regions due to dry winds in comparison to equatorial and polar regions. Dust and salt particles act as hygroscopic nuclei around which water vapour condenses to produce clouds.