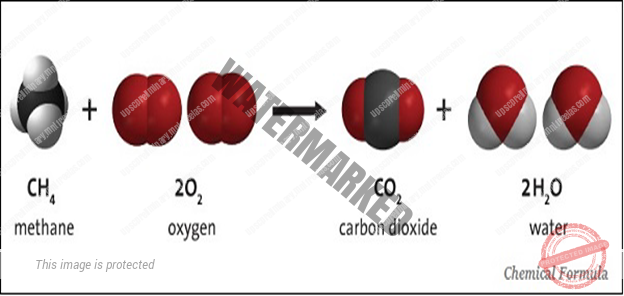
- CHEMICAL FORMULA
- AVOGADRO CONSTANT OR AVOGADRO NUMBER
- STRUCTURE OF ATOM
UNIT 6 – DISTILLATION – PART 3
CHEMICAL FORMULAE
- A chemical formula of a compound demonstrations its constituent elements and the number of atoms of each combining element.
- The chemical formula of a compound is the symbolic representation of its Composition.
- The combining capacity of an element is known as its ‘’
MOLECULAR MASS
- The molecular mass of a substance is calculated by taking the sum of the atomic masses of all the atoms in a molecule of respective substance.
For example, the molecular mass of water is calculated as −
- Atomic mass of hydrogen = 1u
- Atomic mass of oxygen = 16 u
- The water contains two atoms of hydrogen and one atom of oxygen.
- Molecular Mass of Water is = 2 × 1+ 1×16 = 18 u(u is the symbol of molecular mass).
Formula Unit Mass
- The formula unit mass of a substance is calculated by taking the sum of the atomic masses of all atoms in a formula unit of a compound.
AVOGADRO CONSTANT OR AVOGADRO NUMBER
- Avogadro was an Italian scientist who had given the concept of Avogadro Number (also known as Avogadro Constant).
- The number of particles (atoms, molecules, or ions) present in 1 mole of any substance is fixed, and its value always calculated as 022 × 1023.
- In 1896, Wilhelm Ostwald had introduced the concept of ‘mole;’ however, mole unit was accepted to provide a simple way of reporting a large number in 1967.
LAW OF CONSERVATION OF MASS
- During a chemical reaction, sum of the masses of the reactants and products remains unchanged, which is known as the ‘Law of Conservation of Mass.’
LAW OF DEFINITE PROPORTIONS
- In a pure chemical compound, its elements are always present in a definite proportion by mass, which is known as the ‘Law of Definite Proportions.’
STRUCTURE OF THE ATOM
Introduction
- By 1900, it was discovered that the atom was not a simple, indivisible particle, but rather it contains sub-atomic particles.
- J. Thomsondiscovered the sub-atomic particle namely ‘Electron.’
- J. Thomson was the first person who proposed a model for the structure of an atom.
- In 1886, E. Goldstein discovered the presence of new radiations in a gas discharge and named them canal rays.
- Another positively charged sub-atomic particle was discovered with experiments of canal rays and named it proton.
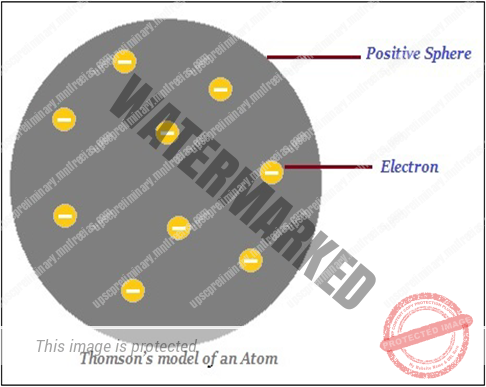
THOMSON’S MODEL OF ATOM
- Thomson proposed that an atom consists of a positively charged sphere and the electrons (negative charge) are embedded in it (as shown in the image given below).
- Further, Thomson said that the negative and positive charges are equal in magnitude. Thus, the atom as a whole is electrically neutral.
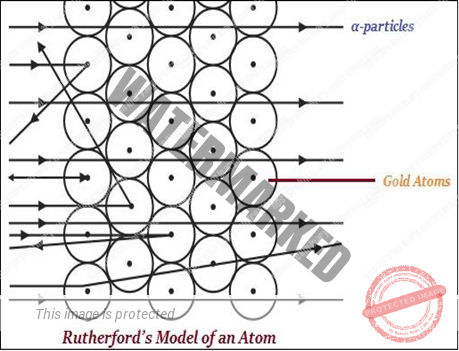
RUTHERFORD’S MODEL OF ATOM
- Rutherford is popular as the ‘Father’ of nuclear physics.
- Rutherford is largely known for his work on radioactivity and the discovery of the nucleus of an atom with the gold foil experiment (as shown in the image given below.
- Rutherford said that in an atom, there is a positively charged center known as the nucleus. Rutherford said that nearly all the mass of an atom exists in in the nucleus. According to Rutherford, the electrons revolve around the nucleus in well-defined orbits.
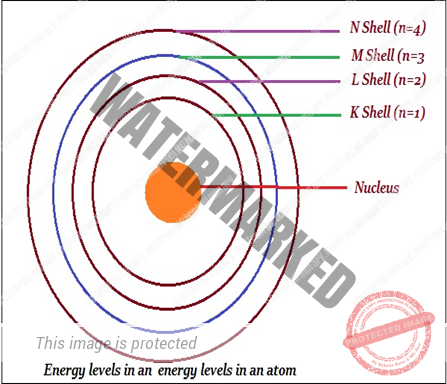
BOHR’S MODEL OF ATOM
- Neils Bohr further extended Rutherford’s model and improved his drawbacks.
- According to Bohr, only certain special orbits known as discrete orbits of electrons, are allowed inside the atom. Bohr said that electrons do not radiate energy while revolving in discrete orbits. Bohr named orbits or shells as energy levels (as shown in the image given below).
- Bohr represented these orbits or shells are by the letters K, L, M, N… or the numbers, n = 1,2,3, 4….
NEUTRON
- In 1932, J. Chadwick discovered a new sub-atomic particle i.e., neutron. Neutron has no charge and a mass nearly equal to that of a proton. Neutrons are present in the nucleus of all atoms, except hydrogen.
Electrons Distributed in Different Orbits (Shells)
- The maximum number of electrons that can be present in a shell is given by the formula 2n2.
- ‘n’ is the orbit number or energy level index, i.e., 1, 2, 3….
- According to the given formula −
- First orbite., K-shell will be = 2 × 12= 2
- Second orbite., L-shell will be = 2 × 22= 8
- Third orbit e., M-shell will be = 2 × 32= 18
- Fourth orbite., N-shell will be = 2 × 42= 32
- Likewise, the maximum number of electrons that can be accommodated in the outermost orbit is 8.
- Electrons are not filled in a given shell, unless the inner shells are filled. It means, the shells are filled in a stepwise manner, starting from inner shell to outer shell.
VALENCE
- The electrons, those are present in the outermost shell of an atom, are known as the valence electrons.
- According to Bohr-Bury model, the outermost shell of an atom can have a maximum of 8 electrons.



[pvc_stats postid="" increase="1" show_views_today="1"]
