- PROPERTIES OF MATTER
UNIT 9 – PHYSICS - PROPERTIES OF MATTER – PART 1
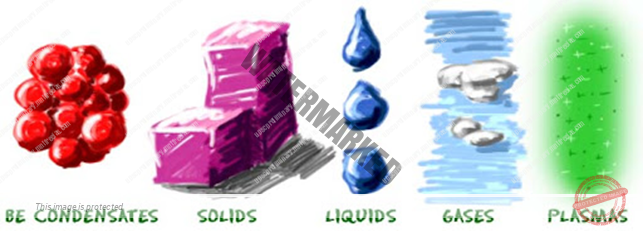
PROPERTIES OF MATTERS:
A matter can neither be created nor it can be destroyed but it can be transformed from one state to another. Matter is made of basic building blocks commonly called elements which are 112 in number. The matter is made of only one kind of element then the smallest unit of that element is called an atom. If the matter is made of two or more different elements, then the smallest unit of matter is Called a Molecule.
Molecule is defined as the smallest unit of matter which has independent existence and can retain complete physical and chemical properties of matters.
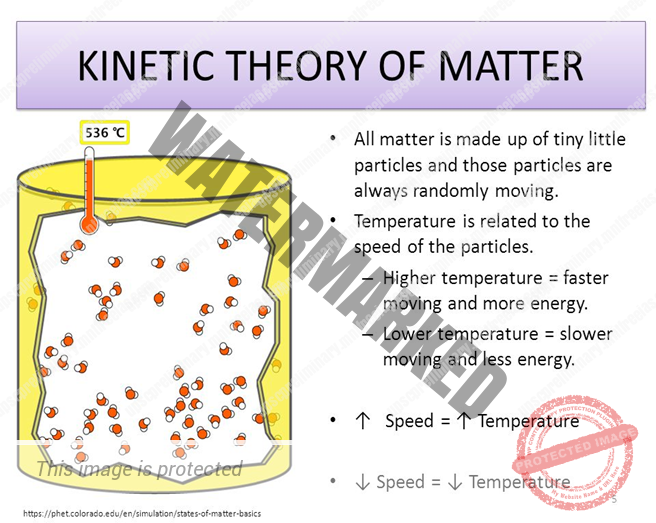
According TO KINETIC THEORY OF MATTER:
(i). Molecules Are in The State of Continuous Motion in All Possible Directions and Hence They Possess Kinetic Energy Which Increases With The Gain Of Heat Energy Or Rise In Temperature,
(ii). The Molecules Always Attract Each Other,
(iii). The Force of Attraction Between the Molecules Decreases with the Increase in Intermolecular Spaces
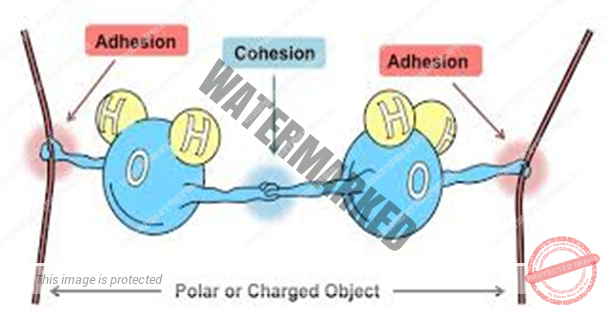
The molecules always attract each other. The force of attraction between the similar kinds of Molecules Is Called FORCE OF COHESION whereas the force of attraction between different kinds of molecules is called force of adhesion.
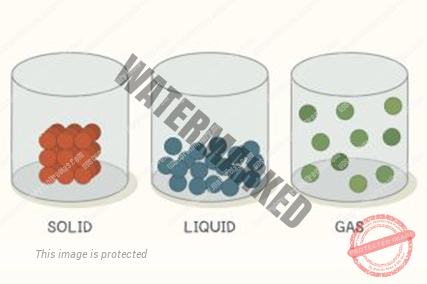
- In case of solids, the intermolecular space being very small, so intermolecular forces are very large and hence solids have definite size and shape.
In case of liquids, the intermolecular space being large, so intermolecular forces are small and hence liquids have definite volume but no definite shape.
In case of gases, THE INTERMOLECULAR SPACE being very large, so intermolecular forces are extremely small and hence gases have neither a definite volume and nor definite shape.